Have you ever seen a label on the packaging of your catheters that warns about DEHP? It’s natural to feel some concern or confusion about this warning.
Since one of 180 Medical’s values is education, we want to let you know more about DEHP and your catheter options.
What is DEHP?
DEHP, which is an abbreviation of di-(2-ethylhexyl)phthalate, is a manufactured chemical plasticizer that is often a component in vinyl or plastic products. DEHP is colorless and has almost no scent. Its purpose is to help soften plastics and make them more flexible.
For example, DEHP and other phthalates are in products like:
- Shower curtains
- Flooring
- Upholstery
- Swimming pool liners
- Plastic toys
- Automobile interiors
- Wire coverings
- Plastic food containers
- Cosmetics, deodorants, lotions, and fragrances
- Some types of medical supplies, like tubing and storage bags

Is DEHP Harmful?
According to the Proposition 65 Warnings website, research has determined that DEHP exposure may be related to the development of cancer, the reduction of testosterone and sperm count, respiratory problems, and other issues such as birth defects.
Due to the wide use of phthalates in everyday products, exposure can happen through skin contact, breathing, or ingestion (drinking or eating it).
Some sources indicate that repeated exposure to DEHP may be harmful.According to the Agency for Toxic Substances and Disease Registry (ATSDR), prolonged exposure to DEHP could impact the male reproductive system and fertility levels. However, this may be primarily due to oral exposure.
Also, based on some specific studies, the Environmental Protection Agency (EPA) has determined that DEHP may be a potential human carcinogen, which means it might cause certain types of cancer.
To date, the exact health impacts of using medical devices with DEHP remain unclear.
Why is There a Warning Label on My Catheters?
Proposition 65, also known as the Safe Drinking Water and Toxic Enforcement Act of 1986, is a law that requires California to publish listings of chemicals that may possibly cause harm to health.
Thanks to this law, you have an opportunity to understand what is in some of the products you use. This is why catheter manufacturers must display warning labels on their vinyl catheter products if they include DEHP.
You may be able to reduce your risk of adverse effects by minimizing your exposure to DEHP. For instance, you could choose to use glass food containers instead of plastic.
Ultimately, you get to make the decision on whether or not to use intermittent catheters that contain DEHP.
Is DEHP in Catheters?
Now, you might be wondering if DEHP is in all vinyl or PVC catheters. The short answer is no, it’s not in every vinyl catheter on the market today.
Some catheters contain DEHP while others do not. It ultimately depends on the catheter brand as well as the catheter type.
How do I know if the catheters I use contain DEHP?
If you’re using a catheter with DEHP, you will likely see a warning label on your catheter packaging.

This is required labeling by law through the California Office of Environmental Health Hazard Assessment. You can learn more about Proposition 65 warnings at the Proposition 65 website.
Why is DEHP in my catheter?
If you’ve noticed that DEHP is in the current catheter type you’re using, you may wonder why. As mentioned above, DEHP is a plasticizer that helps soften certain plastics.
There are a few different types of catheter materials, including red rubber latex, silicone, and PVC (vinyl). DEHP is most common in PVC catheters, although it isn’t in all of them.
So why is DEHP in some PVC catheters? Because PVC can be fairly rigid at room temperature, DEHP is used by some manufacturers to soften the material. This makes vinyl catheters a bit more flexible to aid with insertion during catheterization.
However, now that more information is being discovered about DEHP, some catheter manufacturers are choosing other components to soften their PVC catheters instead of DEHP.
Am I At Risk If I Use a Catheter with DEHP?
Because intermittent catheters are in the body only for short increments of time, exposure to DEHP would likely be minimal.
However, it’s important to understand that there may still be health risks of DEHP in catheters. Researchers still don’t know enough about the long-term health effects of phthalates on the human body.
That’s why we’re providing this information so you can make an informed decision about whether or not you want to use catheters with DEHP. The good news is that you have plenty of DEHP-free catheter options through 180 Medical.
Does 180 Medical Have Catheters Without DEHP?
Yes, we proudly offer one of the widest varieties of brands and types of urinary catheter supplies on the market today, including DEHP-free catheters. Because we want our customers to have a catheter that fits their individual needs and preferences, we make it a point to offer plenty of options from all the top brands on the market.
Also, we carry options made without BPA or DINP, which are other common plasticizers. For instance, Cure catheters are always made without DEHP, DINP, BPA, or natural rubber latex.
At 180 Medical, we like to emphasize the importance of customer choice. We believe you should be able to try out and decide which catheter works and feels best for your needs and preferences, based on your doctor’s prescription and your insurance’s catheter coverage.
Which Catheters Without DEHP Are Available?
You can choose from a wide variety of types and brands of catheters without DEHP at 180 Medical. We’ll list a few popular catheter types made without DEHP below, although you can also check out our online catheter catalog for a complete listing.
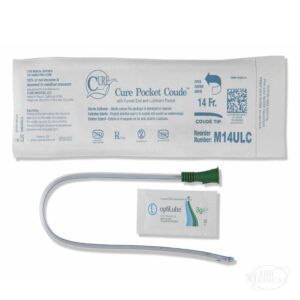
Straight Catheters Without DEHP
Straight intermittent catheters come uncoated and ready for the sterile, water-based lubrication of your choice. If you’d like to try out a DEHP-free straight catheter, here are just a few of the many options available at 180 Medical.
DEHP-Free Hydrophilic and Pre-lubricated Catheters
If you prefer a more ready-to-use catheter, such as a pre-lubricated catheter or a hydrophilic catheter that activates with water, you also have some great options.
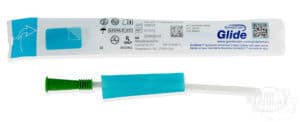
For example, the GentleCath Glide Catheter provides a clean, smooth catheterization with less mess and no DEHP, thanks to its unique FeelClean Technology™.
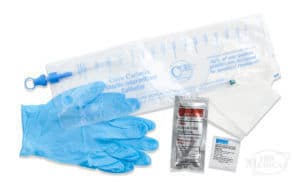
Closed System Catheters Without DEHP
Closed system catheters are often pre-lubricated and come in an integrated urine collection bag, offering a truly touch-free catheterization. These catheters may also come with additional insertion supplies such as gloves and antiseptic wipes, depending on the specific product.
If you prefer a DEHP-free closed system catheter, here are some of the many options available at 180 Medical.
Are GentleCath Catheters DEHP-Free?
Yes! Given the potential risks of phthalates like DEHP, Convatec has decided to begin replacing DEHP with non-toxic plasticizers in their GentleCath catheters.
Whether you need pediatric catheters for kids, closed system catheters, hydrophilic catheters, or straight catheters, GentleCath can be trusted as a comfortable and efficient DEHP-free brand.
These are just a few examples of the many brands and types of catheters available to you when you choose 180 Medical. Contact our Catheter Product Specialists today. We’ll help you find a DEHP-free catheter that’s just right for you!
Reach out to us today!
Disclaimer: This post is for educational purposes only and is not medical advice. For further questions regarding DEHP and its effects on your individual health, please see your prescribing healthcare professional.
For full information regarding DEHP, see the Toxicological Profile for DEHP, published by the California Environmental Protection Agency.